World's Lightest and Lowest Density Solid
Have you heard about the World's Lightest and
Lowest Density Solid?
Its Name is Aerogel.
This piece features a mass of just 1.22 grams that's only sometimes the mass of the identical volume of air, which sort of is sensible because it's 99.8% air. In fact, some aerogels are so light that if I removed all the air from them, they'd be less dense than air. I've got long been fascinated by aerogel so I actually flew intent on AerogelTechnologies in Boston to seek out why was aerogel invented.
1) You know that How is it made? And also
2) Why is it such an honest thermal insulator and what's it used for?
- Okay
To demonstrate the insulating power of aerogel so up here we've two setups:
- one with a glass Petri dish, and
- also the other one with aerogel on top.
- Both are the product of silica, but with very different physical structures. Now to possess a glance at this experiment, we've got a FLIR T1020 which may see temperatures up to 2,000 degrees Celsius. It feels like it's getting pretty hot. I'll be able to see that the glass is getting really hot already. And after just a minute: - It's beginning to smoke. Over here, the Petri dish also cracked under the thermal expansion. So now let's try the aerogel.
3) So how is aerogel invented back in?
Back in 1931, a bloke named Professor Samuel Kistler had a bet together with his colleague Charles Learned.
I mean, they're mostly liquid, but it's embedded within this 3D solid structure. So if I think that of a gel, like jello incorporates a skeleton with nano-sized pores that provides its rigidity, so that's about 1% of the gel.
4) Could I remove the liquid from the jelly, without affecting the solid structure?
I mean, if I only evaporate the liquid out, well, then the solid structure shrinks, because as I remove liquid molecules, they pull on one another and that they pull on the solid structure around them, basically crumpling it from the within. Now Samuel Kistler solved this problem in two ways:-
First, he realized I'll replace one liquid with another inside the jelly just by washing it thoroughly. So I'll swap out, say, water, for alcohol. And then, if I am taking the jelly and put it in an exceedingly high-pressure vessel called an autoclave, and by heating it to the high-temperature, at a high-pressure point called the crisis of the liquid, that liquid transformed into a semi-liquid, semi-gas called a supercritical fluid. At this time, there's not a distinction between liquid and gas. Those molecules aren't any longer pulling on one another. So once I've depressurized the vessel, that solid skeleton, that 1% of the mass of the gel, is left behind intact, aside from where there was liquid within the pores before is now gas, which solid skeleton, that nanoporous solid is what we call aerogel.
Kistler published his findings in Nature in 1931. It's getting pretty hot as I'll see through the thermal camera at three minutes. So we're gonna pull out a thermocouple and just check the temperature underneath.
Like, underneath the aerogel, and see what the flame temperature is?
Exactly, That convective heat is moving up and round the aerogel, So I see the thing is getting sandwich and by 4 minutes, It is still pretty good though, considering how easy it's to melt chocolate. Now I am able to put my finger here, but carefully, it is not that it's hot, it's that it's brittle. But, it's got totally cool to the touch. I made aerogels out of all kinds of things. I made them out of eggs. I made them out of rubber, out of nitrocellulose. And, included in there was silica. Actually, right here on the table, I've got some samples of some silica gels. This is a wet colloid, It's quite rubbery so I can just, carve out a bit. It's 97% alcohol inside its pores. so the remaining 3% solid is amorphous silica. It's reasonably rubbery. Not that strong.
So was I cracking it there or was it already kinda cracked? It's totally easy to interrupt. It is very crumbly, the following step is to interchange the alcohol within the gel with liquid greenhouse gas. I am not on the point of seeing liquid CO₂. Liquid CO₂ has the advantage of being non-flammable, plus it's got an occasional critical temperature. I see it flooding in there. I'll clearly see that it is so much cooler on top.
5) Can you guess what temperature is it on the bottom?
- 600 degrees Celsius, that's 1250 Fahrenheit straight away.
Once the liquid CO₂ has filled all the pores of the gel, it is time to require it supercritical. It was, I'd say, a sort of spiritual experience the primary time that I saw a supercritical fluid. I like what quantity you're into these autoclaves. I really like aerogels to create a supercritical fluid, we can heat this with a hairdryer actually. As we approach the crossroads, the surface of the liquid becomes quite blurry. I'll speed it up so I will watch the surface disappear altogether. I am now viewing the supercritical fluid of CO₂.
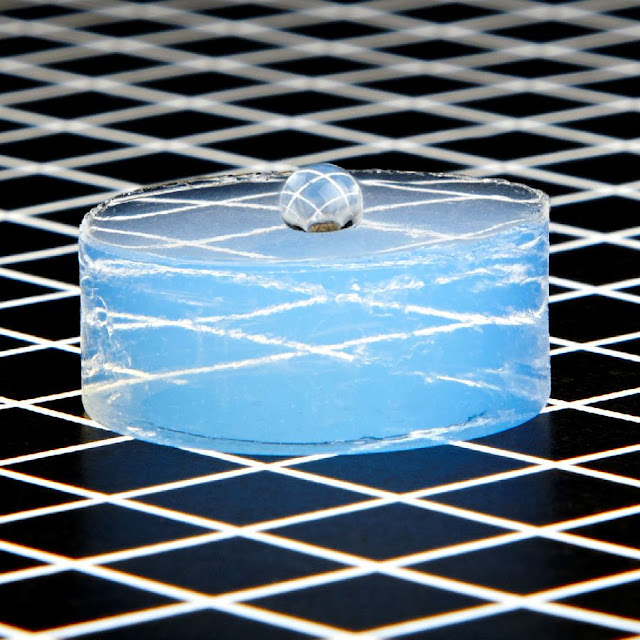
During this state, the CO₂ is often vented without affecting the solid structure. If I examine aerogel on a lightweight background, it's almost impossible to determine, because it's pretty transparent. But when I take a look at it on a darker background, then I will see that it's a small bluish color. And, it's bluish for the identical reason that the sky is blue, because all those tiny little nanoscale structures, they scatter the sunshine in keeping with Rayleigh scattering. And, the intensity of sunshine scattered is proportional to1 over wavelength to the ability of 4, which suggests it scatters shorter wavelengths, like blue, rather more than it scatters yellow or red. And, for that reason, aerogel looks opaque within the ultraviolet and transparent within the infrared.
Now,
6) what does one think this can look like if I held it up to the blue sky?
and
7) What does one thing we might see?
and
8) Would it look ultra blue?
No, it's yellow. and that is because the aerogel is really scattering out that blue light, and then what passes through and makes it to our eyes is that the longer wavelengths just like the yellows and oranges. It's basically the identical effect as gazing a sunset once I see the yellows and oranges of a sunset, it's because the blue light has already been scattered out by the atmosphere the sunshine had to pass through before it reached my eyes. So effectively gazing aerogel against the blue sky is like watching a conveyable sunset. The nanoscale pores of the aerogel also are what makes it such a decent thermal insulator.
9) Does it really hot?
- It's definitely hot.
10) Why does it need insulation?
The electronics, because they do not want the electronics to urge cold during the cold nights on Mars. NASA has also put aerogel to more exotic uses, notably to catch dust from a comet's a part of the Stardust mission, that the particles were traveling about 6 kilometers per second relative to the aerogel, So once they hit the aerogel, because the aerogel's very density material, a very-very porous material, the particles actually enter the aerogel, and as they travel through the aerogel, they basically break apart the network that creates up the aerogel and that they lose energy within the process and eventually, come to a stop.
This is often good for capturing particles, because if a particle-like that were to hit a solid surface then it just stops, I know, immediately. It just vaporizes. So should I expect to work out aerogel in our everyday lives anytime soon?
One in every one of my running jokes is after they build skyscrapers in Antarctica and they'll use aerogel as thermal insulation.
I'll really care about now that how thermal efficiency might be so cold there. So rather than having, I know, ten feet of fiberglass insulation, I may have six inches or something of aerogel. Scientists are currently engaged in reducing costs and increasing durability and that is true but they are doing have some elasticity. So it's not hard to interrupt. I've already made lots of progress.
For example, the original silica aerogel is hydrophilic. Now, this can be a hydrophilic aerogel.
11) So once I've done this, is that piece of aerogel ruined now? What do you think?
There are ways to form it waterproof. So if you wish to determine that and every one the opposite next-generation aerogels, then subscribe to the web the site and this could be the beginning of an aerogel trilogy.






Comments
Post a Comment
Please do not enter any spam link in the comment box